Gallery opened: Aug 2005
Updated: 7 Nov 2016
More on sulphur dioxide




Unusual Working Fluids |
Gallery opened: Aug 2005 |
The Power section of the Museum of Retrotech contains many unusual engines- for some of them, their interest is in the choice of working fluid. Steam and air (the latter as in the internal combustion engine) reign supreme today, for very practical reasons. Both are chemically stable, non-poisonous, and cheap. However, in the past, several other fluids have been tried. Some of these have their own galleries in the Museum; this now includes mercury:
|
|
Liquids that vapourise easily, when used in a stand-alone cycle, are not more efficient than water as a working fluid. Quite the reverse. See: Carnot's Law on the thermodynamics page.
The use of working fluids other than water goes back to the early history of heat engines. Consider:

THE WORKING FLUIDS OF COLONEL TORRENS: 1830
According to The History and Progress of the Steam Engine by Elijah Galloway and Luke Hebert, in 1830 Lieutenant-Colonel Torrens of Croydon was, like the Brunels with their 'gaz' idea, impressed by the high pressures that could be obtained when a liquefied gas was confined and vapourised by heat. A modest amount of heat could produce pressures of 40 atmospheres or more (approx 560 psi) which in the those days was considered extreme. This does not of course mean that a large amount of power is so produced, and naturally power in some form is required to liquefy the gases in the first place. The good Colonel appears to have overlooked this. He planned to use hydrogen sulphide, carbon dioxide, nitrous oxide, ammonia, hydrogen chloride or ammonia.
The boiler and engine were intended to use oil at high pressure as a sealing medium, to prevent leaks of these corrosive and poisonous gases through stuffing boxes, in much the same way as Howard's alcohol engine.

FRENCH WORKING FLUIDS: 1888
It was reported in the French journal Nature for 21 July, 1888, that petrol, alcohol, ether, chloroform, and carbonic acid had all been tried as working fluids. As you can see from the pages listed above this is certainly true, though I have so far been able to sniff out only the sketchiest details of what appears to be the sole attempt to build a chloroform engine. Such an engine would have been interesting to work with; chloroform may be non-flammable, but it is poisonous as well as a (dangerous) anaesthetic.

ODD WORKING FLUIDS TODAY
When you dig a little deeper into the business of unconventional working fluids, you find that some of the more exotic, such as potassium vapour, were once the subject of great interest. The intended application was the generation of power in space, where the heat rejection is at a high temperature, not withstanding the poor Carnot efficiency this implies, because it can only be rejected by radiation. Since there are no plans for large manned spacecraft in the foreseeable future, interest seems to have died.
Another influence is the increasing drive for energy efficiency and renewable sources. Waste heat may be available in huge quantities but at a relatively low temperature, too low to make a steam Rankine cycle viable. Geothermal power frequently has to be generated from water or brine at modest temperatures, which is sometimes used to boil voltaile organic fluids rather than water.
A good example of recent research is a report on seven possible working fluids, produced by the Argonne National Laboratory in 1981. You can read the whole report here (external link)

We will now look at some of the more arcane working fluids:
|
|
|

THE BITUMINOUS VAPOUR ENGINE OF MONSIEUR DE MONTGERY: 1826
Galloway and Hebert's The History and Progress of the Steam Engine refers to an engine mentioned in the French journal Annales de Chemie which was claimed to have worked satisfactorily. It was powered by the pressure of the vapours boiled off bitumen in a furnace- presumably relatively heavy hydrocarbons. After use as a working fluid these vapours were used to fire the furnace.
However, Galloway and Hebert had reservations about it functioning properly, saying "... strong doubts may however be entertained, since nothing has been heard of it for the last three years."
A journal called The Repertory of Arts, Manufactures, and Agriculture claimed in 1823 that the Montgery engine could safely use very high pressures because "it is inclosed in a double case" and that the engine could therefore be forty or fifty times smaller than a steam engine of equal power. This was, of course, pure fantasy.
The Register of Arts, and Journal of Patent Inventions, Volume 3 (edited by Luke Herbert, no less) in 1829 was unimpressed, saying "...not having seen or heard anything of the matter for 3 or 4 years past, we are inclined to think that it was merely one of those evanescent meteor-like schemes. which blaze for a while in the brains of the inexperienced mechanic, and then vanish forever."

SULPHUR DIOXIDE
The boiling point of sulphur dioxide is -10 degC. It is definitely a minority choice when it comes to working fluids. It is poisonous, and in combination with water forms the weak sulphurous acid, H2SO3, not to be confused with the much more corrosive sulphuric acid, H2SO4. Unfortunately sulphur dioxide is easily oxidised by the presence of air or water, giving sulphur trioxide which when dissolved in water gives sulphuric acid.
The greatest use of sulphur dioxide was in pioneering solar power installations, where the low boiling point of the liquid was useful. These applications were directly inspired by the work of Professor Josse in Berlin.
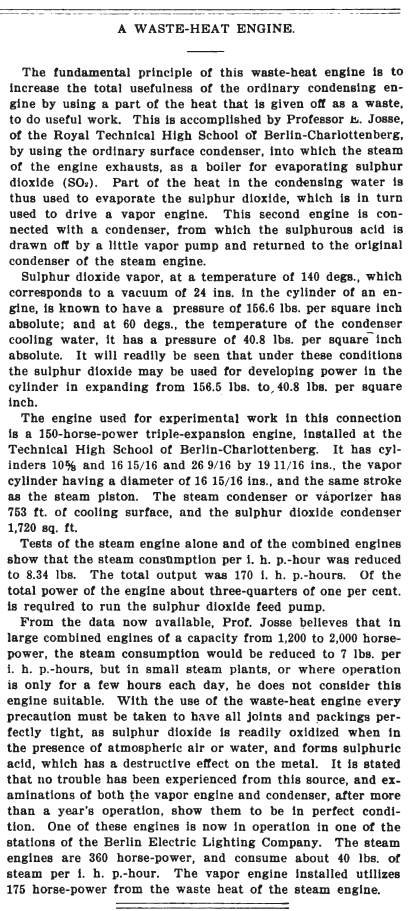 | Left: Professor Josse's sulphur dioxide engine: 1901
|
Henry E. Willsie was a pioneer of solar energy. A Willsie installation had large flat-plate solar collectors that heated water, which could be kept warm overnight in an insulated basin. Liquid sulphur dioxide was passed through pipes immersed in this basin, and it duly boiled to give a high-pressure vapour capable of driving an engine. The sulphur dioxide was then condensed for reuse; quite how, given that the boiling point of sulphur dioxide is -10 degC, is not currently known. Possibly the whole system was pressurised so that condensation could occur at ambient temperature.
In 1904 Willsie built two solar/SO2 power plants. One was a 6-horsepower installation in St. Louis, Missouri, and the other a 15-horsepower system in Needles, California.
Willsie believed that the ability to store energy so power could be generated at night made his system a commercial proposition. Unfortunately he was wrong; no buyers came forward. The long-term cost analysis might have looked good, but it appears potential purchasers were doubtful of the machine's durability; very possibly they thought corrosion from the sulphur dioxide would be a problem. They were also put off by the large amount of machinery required for a tiny power output, and the big initial investment it required. His solar energy company, like other pioneering efforts before it, disappeared.
The use of sulphur dioxide surfaced in a strange place. The crime novel Mystery At Olympia by John Rhode (pub 1935) has an opening sequence set at a motor show in Olympia, and just before the body hits the floor, a salesman gives the following explanation of a new technology called the Lovell Transmission from The Comet Motorcar Company:
"The principle upon which the transmission works is entirely novel. The car is driven, not directly by the engine, but by a turbine, which gives a smoother motion than any reciprocating engine, however many cylinders it might have. This turbine is bolted to the back axle, immediately in front of the differential, [1] thus doing away with the necessity for a long propellor shaft. The space between the turbine and the engine is taken up by this series of steel cylinders."..... irrelevant stuff omitted here. "The engine drives a pump, of a new and highly efficent type. The inlet side of this pump is connected by this copper pipe of large bore to the exhaust end of the turbine The delivery side of the pump is connected by this smaller steel tube to the steel cylinders, which are interconnected. When the car is delivered, these [2] cylinders are full or nearly full, of liquid sulphur dioxide. "The turbine is driven by this sulphur dioxide. When the connection between the cylinders and the turbine is opened, the liquid vapourises, and produces a rush of gas through the turbine, which revolves, and this drives the car. The gas, after doing its work, goes to the pump, where it is once more liquified by pressure and returned to the cylinders." "You will observe that both pump and turbine are jacketted. The compression of the gas in the pump produces heat, and this is utilised in the following way. The pump jacket contains oil, and in this is immersed a carburettor of special design. The mixture, before reaching the engine, is thus heated to such a degree that the petrol is completely vaporised, thus giving ideal combustion in the engine cylinders." [3] "The turbine jacket is similarily filled with oil. But here the effect is exactly the reverse of that of the pump. The vaporisation of the liquid sulphur dioxide produces cold, as in the ordinary refigerator. [4] The cold oil circulates by means of these pipes to the water-jacket, or rather oil-jacket, of the engine, which is thus kept at a suitable temperature." There were only two pedals. "The driver places one foot on each pedal and slowly presses down the left-hand one. The first effect is to admit gas under pressure to the pump, which is caused to revolve, and so start the engine. Further pressure on the pedal releases the brakes. Still further pressure begins to open the connection between the cylinders of sulphur dioxide and the turbine, and the car begins to move." I have added a few notes, see square brackets: 1] This seems to imply that that there is no gearing beyond the usual reduction ratio of the differential. Not good conditions for the efficient operation of a turbine. 2] The cylinders are not mentioned before this in the text. 3] There is nothing new about having a 'hot-spot' in the induction manifold, heated by the exhaust manifold, to aid vapourisation. This however sounds like seriously overdoing it; heating the intake air reduces its density so less mass is drawn into the cylinder and power lost. 4] Refrigerators, including domestic models, in the 1930s used sulphur dioxide (SO2) as a working fluid. It is toxic and a powerful respiratory irritant so leaks could be very dangerous. Apparently when liquefied it acts as lubricant in the compressor, and so there was no need for oil in the system. Before the introduction of freons like R-12N, methyl formate was used as an alternative to sulfur dioxide in domestic refrigerators, such as some models of the famous GE Monitor Top. |
Mr Rhode is essentially describing an automatic transmission rather than the primary power source, which sounds like a conventional petrol engine. It seems to purely a work of imagination. Presumably he thought that because sulphur dioxide worked well in refrigerators it would work well for power transmission, despite its well-known toxicity; it would certainly add a new terror to motor accidents. The concept is unknown to Google.
This odd scheme has nothing to do with the plot of the book. It's quite a good read, though the murder method finally revealed strikes me as implausible. No, it was not sulphur dioxide poisoning.

DIPHENYL OXIDE
An exotic working fluid that once had considerable hopes invested in it is diphenyl oxide, a very stable organic chemical with a boiling point of 258 degC at atmospheric pressure. It was explored as a way of getting heat into a thermodynamic cycle at a higher temperature, but nothing came of it. Oliver Lyle (author of The Efficient Use of Steam) wrote "Diphenyl oxide has been suggested as being more suitable than mercury." in 1958, which sounds as though it had not been tried in practice at that date.
Diphenyl oxide has the structure C6H5-O-C6H5. At room temperature it forms colorless crystals, with a smell of geraniums and phenol; in fact it is sometimes called "geranium crystals". When molten it is a colourless liquid. It is insoluble in water. It is relatively non-toxic as organic compounds go, but I wouldn't sprinkle it on my cornflakes.
Diphenyl oxide has lots of names: diphenyl ether, phenyl ether, 1,1'-oxybisbenzene, geranium crystals, oxydiphenyl, phenoxybenzene, and phenyl oxide are all the same chemical.
Diphenyl oxide is still used in specialised heat transfer applications at high temperatures, sometimes mixed with plain diphenyl; diphenyl is also called Biphenyl, Lemonene, Phenyl Benzene, Bibenzene, and Xenene, and has the structure C6H5-C6H5.

NITROGEN DIOXIDE
Nitrogen dioxide (NO2) is a reddish-brown toxic gas; see Wikipedia At low temperatures it changes into its natural dimer as dinitrogen tetroxide. (N2O4) A cycle that takes advantage of this compresses the N2O4 at low temperature; it is then heated. The higher temperature causes each N2O4 molecule to break into two NO2 molecules in an endothermic reaction. This lowers the molecular weight of the working fluid, dramatically increasing the efficiency of the cycle. The NO2 is expanded through a turbine, and then cooled, causing it to recombine into N2O4 in an exothermic reaction. The N2O4 is fed back by the compressor to continue the cycle, where it takes less energy to compress after its recombination. This is called a dissociative gas Brayton cycle.

ALUMINIUM BROMIDE
According to P K Nag in Power Plant Engineering (3rd edition) aluminium bromide (AlBr3) is a possible high-temperature working fluid that might have the same applications as diphenyl oxide. At a pressure of 12 Bar its saturation temerature is 482.5 degC, well above 187 degC for water. (Mercury is 560 degC) The most common form of aluminium bromide is Al2Br6, which is a hygroscopic colorless solid at standard conditions. Information is scarce but it appears that aluminium bromide might be useful in a dissociation-recombination cycle like that described for nitrogen dioxide and nitrosyl chloride.
Judging by the scarcity of information on the WWW there is not much interest in this substance as a working fluid.

NITROSYL CHLORIDE
Nitrosyl_chloride (NOCL) is a very toxic yellow gas; see Wikipedia, and is also irritating to the lungs, eyes, and skin.
It is not very stable at high temperatures, but that is why it is interesting. A working fluid that dissociates and recombines appropriately can give major improvements in cycle efficiency. Kesavan and Osterle reported at the Intersociety Energy Conversion Engineering Conference, (16th, Atlanta, GA, August 9-14, 1981, proceedings. Volume 3. (A82-11701 02-44) New York, American Society of Mechanical Engineers, 1981, p. 2204-2209) that:
"A study of the Brayton cycle with dissociating nitrosyl chloride (NOCl) as the working medium is reported. With the turbine inlet conditions of the gas in a highly dissociated state (a mixture of NOCl, NO, and Cl2) and the compressor inlet at the combined state (NOCl), the dissociating NOCl cycle shows superior overall performance when compared with the Brayton cycle based on inert gases such as helium. The results of the analysis show considerable potential for reduction in power generation costs through higher cycle efficiencies and smaller component sizes."

RUBIDIUM VAPOUR
Rubidium vapour, as well as sodium vapour and potassium vapour, was investigated for use in long-term manned space travel, where solar cells would become ineffective as distance from the sun increased. A cycle operating at a high heat rejection temperature was essential because the only way of getting rid of heat in space is radiation. There is no convection in a vacuum. Rubidium becomes liquid at 39 degC and boils at 688 degC.
Last Saturday there came one of those rare meetings of a book with the only person in the country likely to buy it. The book is "Design of Space Powerplants" by Donald B MacKay, published by Prentice-Hall in 1963; other odd fluids considered were diphenyl, (it is currently not clear if this meant diphenyl oxide) sulphur, and some substance called Dowtherm-A. It is wholly a theoretical book, but provides some useful insights. There appears to be no discussion of where the heat input to the various cycles comes from, and a nuclear reactor would appear to be the only option.
With the wisdom of hindsight, relying on rotating machinery like turbines for long-term operation in space would be highly optimistic. Turbines are very reliable but they are not wholly maintenance free, and occasionally turbine discs disintegrate, as demonstrated by the Hinkley Point disaster. It is highly unlikely that turbine failure would be survivable in space, what with all those blades flying around. In the event, all this sort of thing was abandoned, and the actual long-term space vehicles were unmanned and had much more modest power requirements. These could be satisfied by Radio-Thermal Generators which have no moving parts and are about as reliable a power source as one could imagine. The two Voyager spacecraft are still being powered by their RTGs; they were launched in 1977.
Interest in metal-vapour turbines has however not evaporated completely (ha!) as a metal-vapour cycle has the ability to absorb heat at higher temperatures than steam and so can be used as a topping cycle to increase power station efficiency.

GALLIUM IODIDE
Gallium iodide has the chemical formula Ga2I6. It is the most common iodide of gallium. The chemical vapor transport method for growing crystals of gallium arsenide uses iodine as the transport agent. Ga2I6 reversibly forms GaI3, and so is presumably of possible use in a dissociation-recombination cycle, but Google is silent on this point. In its GaI3 form it sublimes at 345 degC; the critical temperature is 951K.

METHANOL
Unfortunately, methanol is very toxic. As little as 10 ml swallowed can cause permanent blindness by destruction of the optic nerve. Its low boiling point indicates it would be even less efficient as a working fluid than ordinary alcohol (ethanol).
For more information on methanol see Wikipedia

2-METHYL PYRIDINE/H2O
A azeotropic mixture of 2-methyl pyridine and water. (An azeotropic fluid is one with a single boiling point , even though it is composed of two fluids)
For more information on 2-methyl pyridine see Wikipedia. Note that it is miscible with water.

FLUORINOL 85
"The chosen working fluid is 2-2-2 trifluoroethanol, CF3CH2OH, 85% mol-fraction with 15% mol-fraction of water - known as Fluorinol 85 or F 85.
This azeotropic fluid is supplied by only one manufacturer, and its current demand is low. A significant demand increase could substantially reduce the manufacturing cost of the fluid . If this were accompanied by a substantial reduction of its price, systems based on Fluorinol 85 would become much more economically attractive. At present prices, the cost of a charge of working fluid for a Fluorinol 85 system could be as high as 15% of the initial system cost."
No Wikipedia entry, but there is some chemical info here.
This stuff has been used to make at least one experimental engine, built by the Thermo Electron Corp of Waltham, Mass, which we have come across before, and is described thus:
"The report describes the design of a 3 kw generator set driven by an organic Rankine cycle engine. Power is produced with a turbine-gearbox expander arrangement. The turbine speed of 70,200 rpm is geared down internally to the sealed system to 3,600 rpm so that a low speed shaft seal of proven performance can be used to transmit the turbine output to a standard military generator. The system is completely self-contained with controls designed to maintain working fluid cycle conditions and turbine speed constant and independent of load. The system uses an organic working fluid (Fluorinol-85) rather than water to avoid problems associated with freezing and high temperature lubrication. At full load the cycle operates with turbine inlet conditions of 530F and 480 psia and a condensing pressure of 29 psia (202F)."
Unfortunately the thermal stability of Fluorinol-85 is not perfect. At 600 degF, the annual degradation exceeds 17%. Worst of all, the most significant degradation product is hydrofluoric acid, which is both extremely corrosive and a contact poison, and just about the last substance in the world that you'd want washing around inside an engine.
Thermo Electron Corp now seem to be involved in making scientific instruments, which is no doubt a much safer line of work.

TOLUENE
Unfortunately it's poisonous. For more information on toluene see Wikipedia

FREON R-11
Regrettably, Freon R-11 has the highest ozone depletion potential of any refrigerant, by definition assigned the value 1.0. US production was ended in 1995. This is not promising for a potential working fluid.
For more information on Freon R-11 see Wikipedia

FREON R-113
Also known as 1,1,2-trichlorotrifluoroethane. No Wikipedia entry.
"Fluorocarbon 113 also called FREON(R) 113 or refrigerant 113 is a colourless to water white, non-flammable liquid with a slight, ether like odor at high concentrations. It is noncombustible at ordinary temperature, but the gas will ignite and burn weakly at 1256 degF. Fluorocarbon 113 is chemically reactive with metals such as calcium, powdered aluminum, zinc, magnesium, and beryllium."

HELIUM
has been used as a working fluid in Stirling-cycle engines; it improves their efficiency but it is expensive, and one of the most limited resources on the globe. Hydrogen would be much cheaper and even more efficient but the very small molecules are hard to contain- hydrogen will diffuse through solid metal- and hydrogen embrittlement of the metal parts is also a problem. Hydrogen is also highly inflammable and explosive if (or rather when) it leaks out.
There is a separate page for Helium engines.

SADI CARNOT'S VIEW
Some thoughts from Sadi Carnot, who knew a thing or two about thermodynamics:
"As to the other permanent gases, they should be absolutely rejected. They have all the inconveniences of atmospheric air, with none of its advantages. The same can be said of other vapours than that of water, as compared with the latter. "
From his famous book "REFLECTIONS SUR LA PUISSANCE MOTRICE DU FEU SUR LES MACHINES PROPRES A DEVELOPPER CETTE PUISSANCE" published in Paris in 1824.

  
|