This is a very obscure corner of technology indeed, and I can only offer a few crumbs of information at present. If anyone knows more, I would be most gratified to hear from them.
The boiling point of carbon disulphide is only 46.3°C at atmospheric pressure. Unfortunately, any stand-alone cycle using volatile liquids is doomed to inefficiency because the temperature at which the heat is taken up is low. See: Thermodynamics: Carnot efficiency. However the low boiling point means it can be made to boil by heat rejected from other processes, in a "bottoming cycle" that produces extra useful work.

THE EARLY HISTORY OF DISULPHIDE ENGINES
The info here is as given by William Colwell in his book, tersely entitled 'Triple Thermic Motor'.
The first person known to have considered carbon disulphide as a working fluid was Charles William Siemens who proposed using a mixture of carbon disulphide and steam, but gave no practical details.
The first patent granted for 'Employment of Vapourised Bi-Sulphide of Carbon as a Motive Power' was in England, to Charles Frederick Stansbury in 1854. This was a communication from Bernard Hughes of Rochester, NY, who proposed to fill a boiler with carbon disulphide and heat it in the usual way. Alternatively he suggested a boiler half-full of water, into which the liquid carbon disulphide was injected. Bernard Hughes is unknown to Google.
Arthur Vincent Newton proposed in an English patent in 1856, to use a devil's brew of 1 part coal tar, 8 parts oil, and 8 parts carbon disulphide, which was described as a 'gaseous liquid' which I suggest is a contradiction. It appears that acid was added to convert the carbon disulphide to carbon dioxide, which makes one wonder what the other ingredients were there for.
J O York in a 1856 English patent, proposed to vapourise carbon disulphide by injecting it into a generator heated by steam.
|

THE HUGHES ENGINE: 1856
From the New-York Daily Times for 27 June 1856, Page 3:
"According to the invitation of the Trustees of the United States Motive Power Company, a number of gentlemen, scientific and others, assembled at No. 17 Broadway, yesterday afternoon, to witness a series of experiments with the new Bi-Sulphide of Carbon Engine- Hughes' Patent."
Hughes' Patent has not so far been found.

THE ELLIS ARRANGEMENT: 1872
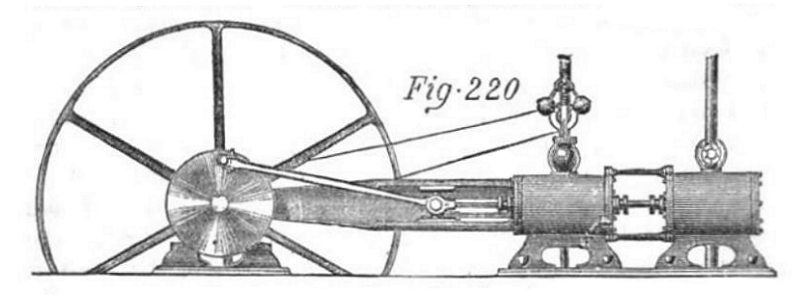 |
| Left: The Ellis engine: 1872
The Ellis system used steam generated by a conventional boiler to drive one cylinder of a tandem engine. The exhaust steam was used to boil "a mixed volatile liquid, consisting principally of the bisulphide of carbon, which boils at 110 degrees F, and... gives a pressure of 65 psi." The resulting vapour was used in a second cylinder, and the vapour then condensed and returned to the bisulphide boiler. The left cylinder is almost certainly the steam cylinder as it carries the speed governor.
|
This is an example of using a volatile liquid in a bottoming cycle, which is not an indelicate mode of transport, but a way of getting more work out of the same amount of heat. Ammonia has also been used for this purpose. Ellis claimed that his system gave 60% more power for the same amount of coal. That might be possible with modern equipment, but seems rather optimistic for the time.
It is stated in the Transactions of the American Society of Mechanical Engineers Vol 7 1886, that Ellis had found a way to make carbon disulphide relatively cheaply. He is unknown to Google and this has not been confirmed.
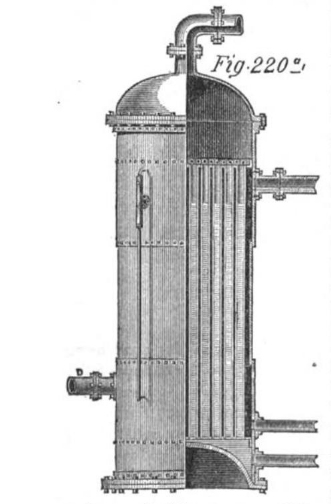 |
| Left: The Ellis bisulphide boiler: 1872
The use of a bottoming cycle is perfectly respectable thermodynamics, unlike the direct heating of liquids more volatile than water; Carnot's Law says unequivocally that a lower boiling point for the working fluid means a lower system efficiency.
The Ellis Vapor Engine Company was set up to exploit the idea, and three companies began the manufacture of Ellis Vapor Engines; the Atlantic Works of East Boston, Poole and Hunt of Baltimore, and the Haskins Machine Co of Fitchburg, Mass. Great savings were reported, but after Mr Poole had nearly lost his life in a vapour explosion enthusiasm waned and activity gradually ceased. Very likely the toxicity of carbon disulphide was making itself felt; according to the Wikipedia page on the substance:
"Around 1900, carbon disulphide came to be widely used in the production of vulcanized rubber. The psychosis produced by high exposures was immediately apparent. (It has been reported with 6 months of exposure). The eminent physician Sir Thomas Oliver told a story about a rubber factory that put bars on its windows so that the workers would not jump out to their deaths."
There is some info on the Atlantic Works here but no mention of carbon disulphide.
There is some biographical info on Mr Poole here, but it does not mention any accident.
There is some info on the Haskins Company here but again no mention of carbon disulphide.
|
 |
| Left: The Ellis Arrangement
"With very little loss", eh? I do hope so, because carbon disulphide is poisonous. See below.
Carbon disulphide and bisulphide of carbon are of course two names for the same stuff.
The only case in which carbon disulphide is used directly in a single cycle appears to be the hazardous method of Fell and Bunster, below.
From Knight's American Mechanical Dictionary, 1881 Edition.
|
The information below comes from the article Substitutes for Steam in the Transactions of the American Society of Mechanical Engineers, 1886, Volume 7, p688, which you can read here.
"In 1872 J H Ellis put forward an engine using carbon disulphide and steam. It was stated that recently (so presumably a year or two before 1886) that two attempts had been made to form stock companies to exploit power generation with carbon disulphide, and that at the present time (1886) there was a company with $25,000,000 of capital seeking inventors."
|
The article Substitutes for Steam was followed by a most interesting discussion:
Mr. John Walker:
"Of the various substitutes for steam mentioned by Mr Babcock, I would advise our members to let bisulfide of carbon alone.
"I happened to be with Messrs Poole and Hunt, of Baltimore, at the time the experiments were made. After the explosion*, I personally aided in assisting Mr Poole, and attending to him and the other parties burnt and otherwise injured. One of the sufferers was overcome with heat and gas. I had the presence of mind (knowing smething about persons being gassed) to take damp sand and put his head into it, until the gas was exhaled from his system."
The experiments were a success until the explosion*; the results were carefully compiled by a young man called John D Isaacs, and I believe published in pamphlet form by Messrs Poole and Hunt. The data were carefully taken, and are very interesting, and my recollections are that they showed excellent results."
"However, there are at least two reasons why we should let bisulfide of carbon alone; First, everything about it smells like a bone-yard: Second, the awful possibility of being blown to Kingdom-come."
|
* "The experiments were a success until the explosion." is not a good outcome for anything.
Mr. F W Taylor:
"Might I ask Mr Walker to explain more thoroughly his method of resucitating men who have been overcome by the effects of gas?"
|
There then follows some more nonsense about breathing through damp sand as a way to eliminate gas from the body.
Mr. Crane:
" I would like to state that the difficulties in refence to the bisulphide vapor are directly dependent upon the work with it is done. I was around the present motor two days, day andnight, and could not tell that there was any bisulphide used. In was kept in the generator and engine right along. It would have been as difficult to set fire to it as it would have been to set fire to a pile of ice. Every receptacle in which the bisulphide was contained was surrounded by a steam-jacket. A steam-jacket is a very good protector. There was no place in the working of the engine where the gas was discharged. The vapor was retained. We cannot admit as engineers that the world does not move on. Steam itself made a great many enemies because it cast some smoke over the green fields of England at first, and frightened the cows. Electricity has paralysed a great many men who did not know how to go about their work, and so it has been with the substitutes for steam. Men have experimented and taught their successors how to manage that motor."
|
This seems remarkable complacency when you consider that an explosion DID occur, and so there must have been an escape of bisulphide vapor. Also, I never heard that an electric shock can paralyse you, but it seems breathing bisulphide vapor could.
Mr. John Walker:
"Some trouble was found, at the time to which I refer, with the bisulphide of carbon itself. The bottom of the condenser would occasionally be filled with small crytstals, mixed with the lubricant that went from the condenser from the engine. These crystals seened to have formed during the condensing process. I might add also, that all the working parts of stop-valves, pump and engine, that came in contact with the bisulphide of carbon in liquid or gaseous condition, had to be of iron, and the stuffing-boxes were made double- such as are used in ammonia pumps, with a by-pipe in the chamber to carry off any escaping vapor or liquid."
|
This tells us that the double stuffing-box was a well-known adjunct to ammonia pumps, presumably those used in refrigeration. This makes it odd that Mr Colwell (see below) was able to get a patent for the same idea in 1885.
Mr. Crane:
"There is a little exhaust-chamber in the stuffing box of the present motor- an ordinary stuffing-box was used. Any vapor or liquid which attempted to follow the rod out was sucked right away to the condenser; of course, nothing was lost."
|
More on the double stuffing-box.
In the discussion various names emerged as linked with carbon disulphide.
Henry L. Gantt and Dabney Herndon Maury produced an article called "The Efficiency of Fluid in Vapor Engines", in Van Nostrand's Engineering Magazine for July-Dec 1884, p413-433. You can get it here.
They examined water, alcohol, ether, carbon disulphide, and chloroform as working fluids. I quote:
"From the above it is seen that steam requires the smallest cylinder to produce a given power, if worked between the limits of pressure we have taken. It also more efficient than any of the vapors, except choloroform."
The final conclusion is that "...it seems certain that none of the non-aqueous vapors will ever successfully compete with steam."
Henry L. Gantt is best known for inventing the Gantt Chart for project planning.
The Transactions describe Laboreau, who also used the double stuffing-box: "...many years previously." This sounds like a French worker, but nothing has so far been found. There is also discussion of the Keely and Paine motors. The first was definite scam; the Paine motor has so far not been found, though there is an article titled "Paine's Electric Motor on Exhibition" in Scientific American May 26, 1855 Volume 10, Issue 37. It is behind a paywall.

This description appeared in an Australian newspaper in 1871, no doubt copied from an American paper:
UTILIZING WASTE HEAT FROM STEAM ENGINES AND BOILERS.
"Mr. J. H. Ellis, of Springfield, Vt, has recently made some interesting experiments in utilizing the heat that escapes in the exhaust steam from engines, and in the
smoke from steam boiler furnaces. He used for the purpose a horizontal tubular steam boiler, 12 inches in diameter and 3 feet long, with thirteen copper flues 1 inch in diameter; the fire-box being under the boiler, and the smoke returning through the flues. He connected with this boiler an engine, with cylinder 15 inches x 20 inches, running 300 revolutions a minute. This engine was geared to a derrick so that it raised a weight of five hundred pounds five feet in one minute. For the purpose of using the escaping heat from this engine and boiler, he placed another upright tubular boiler in the flue of the chimney, the base of the
flue being enlarged sufficiently for the purpose. This boiler was four feet long and nine inches in diameter, and had seven copper flues, 1 inch in diameter,
A spiral coil of copper pipe was placed inside this boiler, of sufficient length to extend from one end to the other ; one end of the coil passing out at the top, and the other end at the bottom of the boiler. The diameter of this coil was 8 inches, over the diameter of the pipe of xvhichitwnsmado was J second The upper end of this coil was connected with the exhaust pipe of the engine, so that the exhaust steam was compelled to pass through the coil to escape into the atmosphere. The boiler was filled with the bisulphide of carbon (which boils at about 100" Fahrenheit), and it was connected with another engine, of the same size and style as
the one used with the steam boiler, and geared to a derrick in the same manner.
Having raised the pressure in the steam boiler to do lbs., the steam engine unsalaried, raising with the derrick a weight of 500 lbs., the exhaust steam passing through the coil of pipe in the bisulphide boiler in; the manner described. In five minutes after the steam engine commenced running, the pressure in the bisulphide boiler went from 5 to 30 lbs. to the inch. At this time the second or bisulphide engine was started, geared tenderness, and commenced raising a weight of 500 lbs. in the same manner that the steam engine was doing The two engines were kept running simultaneously two hours, and during this time the steam engine made 38,000 revolutions, and raised 680 lbs. 460 feet ; while the bisulphide engine made 44,000 revolutions, and raised 500 lbs. 528 feet. The pressure in the steam boiler ranged from 30 to 70 lbs, to the inch, averaging about 45 lbs. and the pressure in the bisulphide boiler ranged from 30 to 60 lbs., averaging about the same as that of the steam boiler. The temperature of the smoke on leaving the flues of the steam boiler did not exceed 360 degrees during the War. The exhaust steam was perfectly condensed in the coil, and all its latent heat imparted to the fluid that surrounded it and the temperature of the water discharged from the cost did not exceed 108-, being reduced to that point by the cold bi-sulphide constantly pumped in at the bottom of the boiler around the lower ends of the coil. The heat of the exhaust steam being applied at the top of the boiler, a pressure of 60 lbs. to the inch was obtained, before the temperature at the bottom of the boiler was raised a single degree. The vapour of the bisulphide of carbon was condensed in a short coil of copper pipe, immersed in a tank of water and pumped back into the boiler continuously during the trial, with no perceptible loss of the material. Presumably meaning the air did not reek of carbon disulphide: Ed
The amount of fuel consumed in getting up steam from cold water, and running the engine during the trial, was 5 lbs, of wood and shavings, 6 lbs. of charcoal, and 12 lbs. of anthracite coal and 60 lbs. of water were condensed from the exhaust of the steam-engine, in the coil of the bisulphide boiler. It will be seen by the above statement of facts, made from data furnished by Mr. Ellis, that the increase of power obtained from a given amount of fuel by the use of the bisulphide boiler was 115 per cent. Mr. Ellis states that owing to the fact that the engines used had no cut-off valves, and had ports too much contracted to exhaust freely, and also because the amount of friction in the derrick gearing, which was new, was very great, the amount of power developed and useful work performed was not as much as it would have been with more perfect engines. But as this bisulphide engine laboured under precisely the same disadvantages that the steam-engine did, the power gained, by the use of the former was not affected thereby.
Source: The Sydney Morning Herald (NSW : 1842 - 1954) Mon 22 May 1871, Page 6
The text has clearly been OCR-ed at some point and a few words are lost.
|

THE METHOD OF FELL & BUNSTER: CARBON DISULPHIDE AND GLYCERINE: 1877
In this scheme carbon disulphide is heated directly in a boiler that was part-filled with glycerine. Given the inflammability of carbon disulphide this strikes me as a hazardous process.
 |
| Left: The Fell & Bunster patent
Thomas Fell and Henry Bunster explain in their patent that all previous attempts at carbon disulphide engines have failed because when the hot CS2 vapour contacts metal in the presence of water, it reacts, leaving the sulphide of the metal and liberating carbon. (I have no idea if this is correct- I am just quoting them) Their idea was to use glycerine to heat the CS2 vapour and cover the metal parts with a thin protective film. They say "The properties of bisulphide of carbon are well known. It is easily evaporated into a dense vapour, the gravity of which is 2.621, by a heat of 110° Fahrenheit. The latent heat absorbed for vaporization is about 280° Fahrenheit, that of steam being nearly 1000°, or a saving in fuel of 71 per cent."
The saving of fuel is of course thermodynamically infeasible, and saying that latent heat is measured in units of temperature does not inspire confidence.
A is the boiler, part-filled with glycerine, B the condenser, C the boiler feed pump, and E "an ordinary engine". The liquid CS2 was sprayed onto the surface of the glycerine in the boiler. The safety-valve on top of the boiler does not exhaust to the air (for which we may be thankful) but connects with the exhaust pipe from the engine to the condenser.
|
The patent gives no clue as to whether this scheme was ever constructed and tested, but F & B are unknown to Google apart from this patent, so probably not.
The F & B patent mentions the exploits of Bernard Hughes, (see above) who in 1854 patented a method of injecting carbon disulphide into water, and using the resulting vapour to propel machinery.

COLWELL'S CARBON DISULPHIDE MOTOR: 1879
The big name in carbon disulphide engines is William S Cowell, of Pittsburgh, Pennsylvania.
William S Colwell was granted US patent 220,220 for a 'Motor and apparatus for utilising it' in October 1879. This appears to have been his first foray into the world of carbon disulphide. One of the patent claims was for coating the vessels used with tin to prevent corrosion from the carbon disulphide.
In this design the carbon disulphide is heated not directly in a boiler but indirectly by steam. The steam boiler A passes steam to the disulphide boiler by pipes D and D', which steam-jacket the disulphide cylinder H. The disulphide vapour passes to the engine cylinder H via a pipe t, which is concentrically inside pipe D'. The exhaust disulphide goes via pipe u to the disulphide condenser at F, which is supplied with both cooling water and forced to agitate the water.
As is often the case, the patent text is long and complicated, but having pieced through it I have not found any claim that this machinery will be more efficient than a much simpler conventional steam engine, though it does claim 'great economy'. There is a passage about the heat from the exhaust carbon disulphide being returned to the vapour generator, but unless the heat is being pumped uphill by the expenditure of energy, this is forbidden by the Second Law of Thermodynamics.
 |
| Left: Colwell carbon disulphide motor: 1879
- A = steam boiler
- B = steam dome
- C = steam safety valve
- D = steam pipe from boiler
- F = disulphide condenser
- f = boiler feed pump (water)
- A' = disulphide boiler
- H = engine cylinder
- 7 = level gauges
- 8 = water test cocks
This diagram shows the steam boiler with its coal fire very close to the carbon disulphide plumbing, which would be highly dangerous. This was presumably just to get it all in one diagram, and one hopes that if this machinery was actually built- and it's starting to look as though it was- the steam boiler was a long way away.
From US patent 220,220 Oct 1879
|
This passage in the patent text shows that Colwell was fully aware of the inflammability of carbon disulphide:
"The advantage of the method and means hereinbefore described for evolving the bisulphide of carbon into a vapour for a motor without the presence of fire at or near the vaporizing chamber is a very important feature of my invention, the advantage and value of which cannot be overestimated..." I quite agree.
|
However, Colwell does not specifically warn that carbon disulphide is highly inflammable, and nowhere in the patent does he mention that it is highly poisonous. However, I suggest it is significant that he took out US patent 313,180 "Means For Packing Piston And Valve Rods" which concerns itself with preventing leakage of a working fluid: "My invention relates to the packing of piston and valve rods of engines, particularly bisulphide-of-carbon engines". Leakage was rendered innocuous by arranging a vacuum chamber inside the packing which would intercept and waft away any leakage before it reached the outside world. I suggest that since Colwell went to the trouble and expense of a patent, he was battling a serious problem..

COLWELL'S CARBON DISULPHIDE LOCOMOTIVE: 1879
This remarkable proposal for a locomotive driven by carbon disulphide vapour is taken from US patent 225,689, filed in October 1879, by William S Colwell of Pittsburgh, Pennsylvania. Like the motor above, it uses steam to heat the liquid carbon disulphide indirectly.
 |
| Left: The Colwell locomotive in section
Colwell says in his patent that carbon disulphide vapourises at 118 degrees Fahrenheit, (the modern figure quoted above is equivalent to 115.3 degF) and will generate a pressure of 186 psi when heated to 295 degrees F.
The design is as follows: B is a conventional firebox surrounded by water-space C, connected to two small water boilers D with conventional fire-tubes e; a Y-piece f connects them to chimney g. The water-space is fitted with a steam dome h, pressure gauge j, and safety valve k, all of which are conventional.
s' is an air-cooled condenser for the vapour from the engine cylinders.
From US patent 225,689, filed Oct 1879
|
 |
| Left: The Colwell locomotive in section
The two lower boilers D generate steam which passes through the tubes m and r in the carbon disulphide boiler l at the top, which Colwell calls "the evolving chamber". This boiler is surrounded by another space q, which is filled with a non-inflammable liquid. Colwell seems to have thought this was a sufficient protection against fire, though he does not specify what liquid is to be used; presumably not water as that would be boiled off by the steam tubes. The carbon disulphide boiler has an outer dome s which encloses the inner carbon disulphide dome t. A regulator valve in dome t controlled the flow of carbon disulphide vapour to two outside cylinders with slide valves.
The the inner carbon disulphide dome t has a so-called "safety-valve" that presumably blasted out poisonous and inflammable carbon disulphide vapour when the pressure became excessive; this is perhaps better than an explosion, though maybe not by much, bearing in mind that this valve is only a few feet from the furnace. Quite possibly there would be an explosion anyway.
In his patent Colwell admits to two problems with his exotic working fluid. The first was corrosion of the engine valves and cylinders, the second was loss of the lubricant properties of oil as carbon disulphide reacted with it. He claimed to have solved both difficulties by using finely-ground graphite mixed with oil, as a lubricant. This does seem to indicate that he had at least done some real experiments with carbon disulphide operation.
Colwell claimed his engine would operate "with great economy and perfect safety". Perhaps surprisingly, he lived to take out more patents for carbon disulphide prime movers in 1882 and 1885.
|
It is not currently known if anyone tried to actually build and operate this locomotive; one hopes not. It seems extremely unlikely that they did.

COLWELL AND THE TRIPLE THERMIC MOTOR: 1885
William Colwell's interest in carbon disulphide as a working fluid did not evaporate. He took out US patent 313,176 'Triple Thermic Motor' in March 1885, and US patents 432,510 and 432,511 with the same title in July 1890. This is not the first time I have found two apparently identical patents with adjacent numbers. I have no idea what it means.
The New York Times for 2 March 1884 carried this headline:
ANOTHER WONDERFUL MOTOR; A NEW POWER WHICH MAY SUPPLANT THE STEAM ENGINE.
"CHICAGO, March 1. An announcement is made of the discovery of a new and remarkable motor known as "The Triple Thermic Motor." The new motive power is the vapor of bi-sulphide of carbon. It has been in practical use in driving a 60-horse power engine for six months past in a cement paving manufactory on West 46th-street, New-York."
|
I may be wrong but I seem to detect some world-weariness in: 'ANOTHER WONDERFUL MOTOR'. I suspect that the New York Times was recalling the Zeromotor scandal which was about at the time.
Another unenthusiatic reference to the Triple Thermal Motor was made in Scientific American in 1884. You can read this on the Odd Working Fluid page.
Colwell produced a book to publicise the Triple Thermal Motor. You can see it here. Part of the book is an article which in its conclusion makes some startling claims:
- 1: "Fuel consumption is reduced to one-quarter". Obvious nonsense.
- 2: "Does away loss of power through incrustation and corrosion in boilers." How? You're still boiling water to make steam.
- 3: "There is less liability of explosion." You must be joking.
- 4: "Its adoption by any one person or corporation in any particular line of business will make it necessary for all others engaged in the same line to adopt it or retire from business." Just a bit over-optimistic.
These claims seem to indicate that the author (and I am sure it was Colwell) has by now lost all touch with engineering reality.
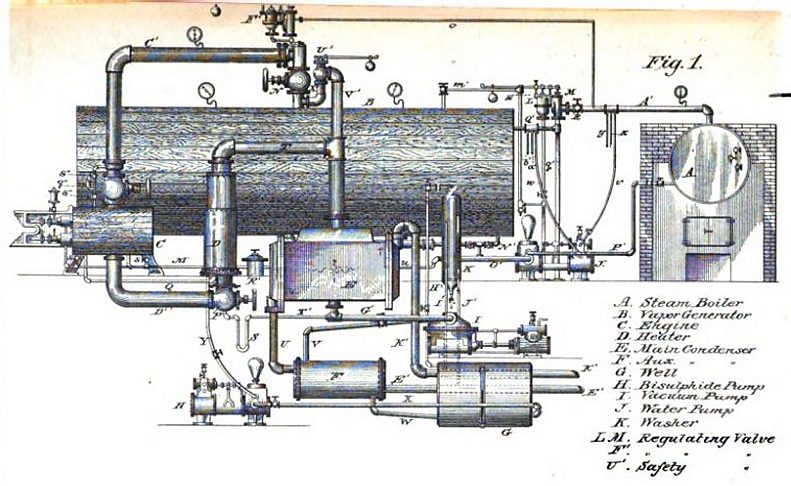 |
| Left: The Triple Thermic Motor: 1883
The arrangement here appears to be much the same as in Colwell's 1879 patent. Steam is raised in the boiler A and passed to the disulphide boiler B. The vapour from this drives the engine C. The same dual-concentric pipework is used.
Note that the disulphide safety valve U exhausts straight into the engine room.
From the book on the Triple Thermal Motor mentioned above.
|
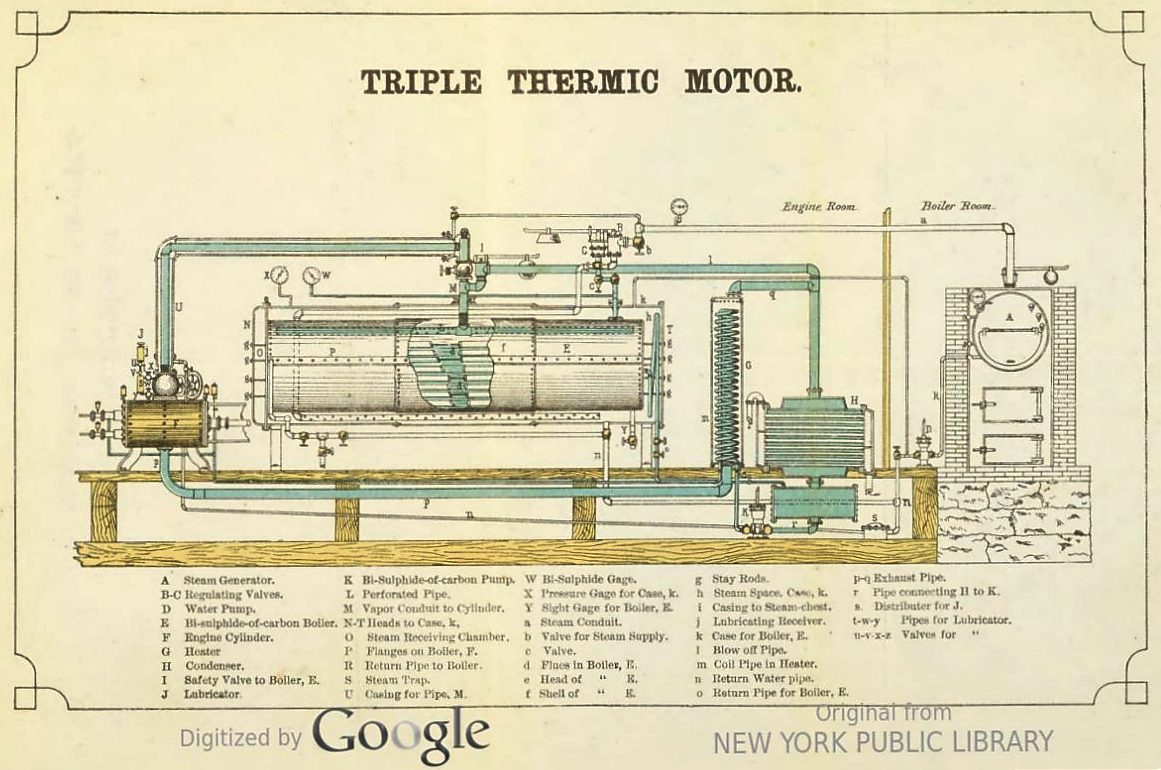 |
|
Above: The Triple Thermic Motor: 1883. From the book on the Triple Thermal Motor mentioned above.
At last there is explicit recognition that it might be better to have the steam-boiler and its fire in another room, in case of disulphide leaks. Especially since the safety valve I again exhausts straight into the engine room.
|
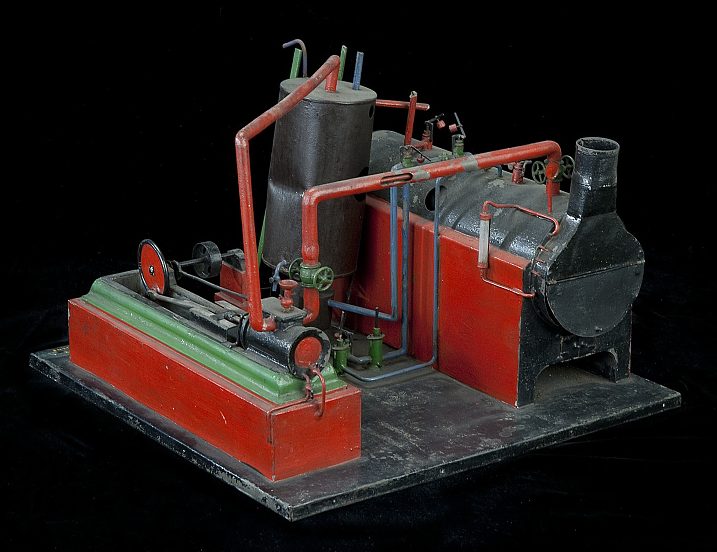 |
| Left: Patent model of the Triple Thermic Motor: 1883
In some cases, applications for patents in the US system were accompanied by models. This is the model that accompanied the 1883 Colwell patent. It looks a bit crude and it seems unlikely it was a working model. Note that the dual-concentric pipes are modelled and one is cut away to show the inner pipe.
Scale unknown.
|
The question remains as to whether Colwell was deluded or a crook. Bearing in mind that: "...no disinterested tests were allowed, and purchasers of stock are said to have been badly stuck." (See on the Odd Working Fluid page) I'm inclined to go with the latter.
From the School of Mines Quarterly, Volume 7 1886 pp210-218
"The first design made in this country for a bisulphide of carbon engine, was presented to the Novelty Iron Works, of this city, about twenty years ago. Previous to this however, trials on a large scale were made in France." See M.Laboureau above.
Nothing has been discovered so far about disulphide engines in France.

CARBON DISULPHIDE FOR ELECTRICITY GENERATION: 1886
Some nifty detective work by the Museum Staff has identified this engine as that of Mr Colwell. Scientific American for 15 Sept 1883 reports a new bisulphide engine by Colwell and locates it at Lowell, Massachusetts. Since the installation comprised 138 arc lights and 21 miles of wire, it must have been a street lighting project. This may be a unique case of a disulphide engine doing a useful job of work, albeit less efficiently than a straight steam engine.
From Mr Thomas S Crane: (who is unknown to Google)
"Having been recently called upon to examine a carbon bisulphide engine operating an electric plant at Lowell, Massachusetts... The plant at Lowell consisted of two 14x28 inch engines coupled together and supplied with CS2 vapor at 80 pounds pressure by two generators containing liquid bisulphide. These generators were jacketed with live steam, as well as the vapor conduit to the engines, and the cylinders and steam-chests of the latter."
"Steam at 40 pounds pressure was furnished by a boiler having less than 800 ft of heating surface (estimated at 60 horsepower) and was fed at a regulated pressure of 20 psi to the jackets of the CS2 generators and at a pressure of 40 psi to the jackets of the engine cylinders and vapor conduits. The CS2 vapor operated in a closed-circuit, passing through a surface condenser and being returned by a pump to the generators."
"The engines operated two lines of heavy shafting and six 25-horse dynamo-electric machines at the time of this examination; and during a greater part of the time furnished a current to 138 arc lights of 2,000 candlepower each, through 21 miles of wire, developing 174 horse-power by the indicator cards. The consumption of coal was 2012 pounds (containing 1634 ponds of combustible, with a new fire) during the entire period of the examination, 8.5 hours."
(Paragraph omitted)
"I would like to ask first whether, in view of these facts, it is reasonable to say that no subsitute for steam can be found to develop greater economy? Secondly, whether any horizontal tubular steam-boiler having less than 800 feet of heating surface is anywhere operating a steam-engine with a load of 174 indicated horse-power?"
Source: Transactions of the American Society of Mechanical Engineers, 1886, Volume 7, p688
|
Well the answer to the first question is YES, very reasonable. Steam is best almost always; that is only not so if you are operating in an extreme environment such as outer space, when potassium vapor may be your friend. As for the second question, I have no idea.
On the history of elecric street lighting in Lowell:
"In 1888 alone, 33 electric lights were added to the 119 that had been in place in Lowell when the year began, not including the eight electric lights on Fort Hill Park by 1888, which ran during the summer months. The number of gas street lamps continued to decline as Lowell progressed into the twentieth century. By 1909, Lowell had outsourced the care of its remaining gas lights to an outside provider."
There was a Lowell Electric Light Corporation, incorporated in 1881, but whether this was the outfit that hosted Mr Cowell's engine has not so far been determined.
It is a great pity that Mr Crane did not give any practical details. Was the engine surrounded by a constant stink of disulphide? Were the workers showing any signs of distress? See the next section.

CARBON DISULPHIDE AS A SUBSTANCE
There are lots of reasons why using carbon disulphide as a working fluid is not a good idea. As soon as you depart from water or air, you find yourself dealing with something that is expensive, explosive, poisonous, or all three. So what about carbon disulphide?
I don't have any figures to hand for the cost of carbon disulphide, but it is clearly going to cost a lot more than water.
Carbon disulphide evaporates at room temperature, and the vapour is more than twice as heavy as air. Carbon disulphide easily forms explosive mixtures with air and ignites very easily; it is dangerous when exposed to heat, flame, sparks, or friction. Vapors can be ignited by contact with an ordinary light bulb.
Acute carbon disulphide poisoning is very dangerous. Absorption can occur through the skin, by ingestion or by inhalation. In severe poisoning, the subject quickly becomes comatose and death occurs in a few hours, usually due to respiratory depression and convulsions. In less severe cases local irritation, nausea, vomiting and abdominal pain are followed by headache, euphoria, hallucinations, panic delirium, paranoid reactions and suicidal tendencies. In other words, your typical engineering development project. (As noted above, Sir Thomas Oliver told a story about a rubber factory that put bars on its windows so that the workers would not jump out to their deaths)
 |
| Left: Trouble with carbon disulphide: 1896?
Source: The British Medical Journal
|
Another opinion:
"A low boiling point, high toxicity and an exceptional fire hazard make carbon disulphide an extremely dangerous substance; its use should be avoided wherever possible; usually a less dangerous substitute can be found."
From Safety In The Chemical Laboratory" by Pieters & Creyghton, pub Butterworth 1957.
And yet I can remember using it in the chemistry lab at school...
Not for the first time, it occurs to me that water is a really good choice as a working fluid.

















