Updated: 17 June 2011




The Caustic Soda Locomotives |
Updated: 17 June 2011
 
|
The caustic soda was reconcentrated either by transferring it out of the tank and boiling it in open vats, or, rather more conveniently, by injecting superheated steam at a high enough temperature to boil off the water in solution. It sounds like a rather elegant process, and distinctly safer than ammonia, but it had little success.
An interesting point is that these locomotives were always referred to as "soda locomotives". This implies they used "soda" which is commonly understood to mean washing soda, (sodium carbonate) which is much milder stuff than caustic soda (sodium hydroxide). The latter can give you nasty alkali burns. Very possibly this subtle misnaming was designed to mislead the public. Taking a trip sitting next to a ton of hot caustic soda might have made some people nervous...
Information on this system is hard to find, and if anyone out there can point me towards more information, I would be most grateful.
The various soda engines and locomotives below are in chronological order.
THE PERKINS CALCIUM CHLORIDE ENGINE: 1864
Mr Loftus Perkins writes: "I then (in 1864 or 1865) constructed a four horse-power engine worked by a boiler immersed in a bath of chloride of calcium, the exhaust steam being condensed in the salt and all the waste heat from the engine being entirely absorbed, thus making a perfect heat engine. Succeeding so far, the difficulty arose how to get rid of the water absorbed by the salt; this could only be done by distillation, and even if quintuple distillation* is used, the coal consumed could not be less than 1.25 lb per horsepower per hour, whilst a well-constructed high pressure compound condensing engine used less than 1 lb."
From a report in Engineering, April 1885.
THE SPENCE CAUSTIC SODA BOILER: 1874
In Jan 1874, Mr Spence, Jun (which I assume means Junior) proposed to pass the exhaust steam of a high-pressure engine into a solution of caustic soda, which he calculated would be heated to 375 degF. The hot caustic soda was then to be circulated through heating pipes in a boiler to raise more steam. This was proposed as a means of utilising the energy in the exhaust steam rather than as a fireless propulsion system. Since the caustic soda would soon need to be reconcentrated by boiling, using more fuel, it is highly unlikely that the process was economic, and indeed, it never seems to have been heard of again in this context.
From a report in Engineering, April 1885.
THE HONIGMANN CAUSTIC SODA LOCOMOTIVE: 1885
As reported in the British journal Engineering for Feb 27, 1885
The Honigmann condenser, as it was known at the time, was a German development. I believe, but have not yet confirmed, that Honigmann was involved in the manufacture of caustic soda, which would explain his enthusiasm for the system. Early tests were done on a steamer on the River Spree, near Berlin, with some success. Another trial on a Berlin tramway was less satisfactory. The next step was a locomotive.
The Honigmann locomotive was a rebuild of an old passenger train locomotive built in 1862. The boiler was fitted with 730 Field tubes to increase surface area; the total heating surface was 38 m2. The locomotive was tested on a short four-mile branch line between Würselen and Stolberg over ten days; this was a branch off the Aix-la-Chapelle to Jürich line. Eight trains ran each day, each journey lasting 25 minutes. Operation was therefore intermittent, comprising only three hours out of each twelve, and this was unfavourable in terms of economy to both soda and conventional locomotives.
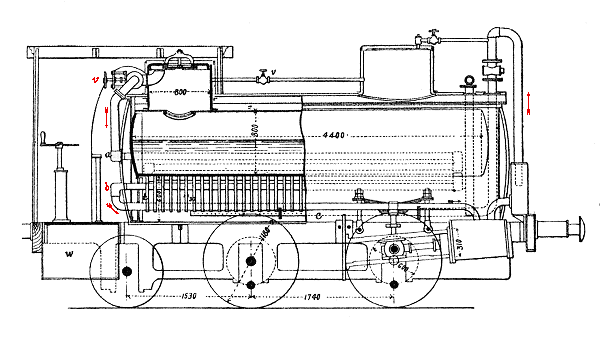 | Left: Section of the Honigmann locomotive.
|
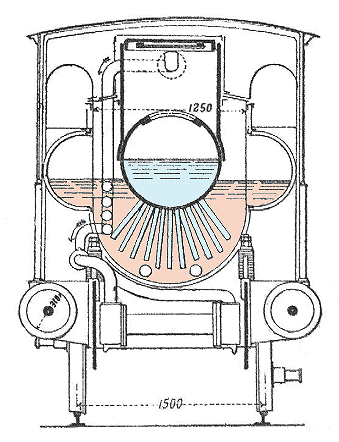 | Left: Section of the Honigmann locomotive.
|
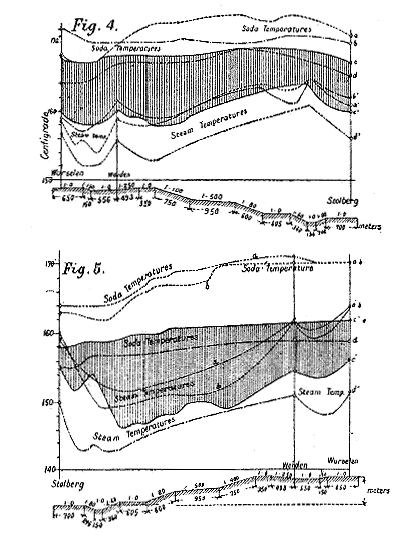 | Left: A record of soda and steam temperatures for the Honigmann locomotive. The graphs are in degrees Celsius.
|
The trial was judged to have been successful. At the time it was reported that Aix-la-Chapelle was to have steam trams with Honigmann condensers, and that two caustic soda locomotives of 45 tons weight had been ordered from Hanover Maschinenbau Actiengesellschaft for use in the St Gothard tunnel under the Alps.
In 1896 it was proposed that a combination of steam storage and caustic soda re-activation on the 'Honigmann' principle would be an effective means of propulsion for submarines. Engineering concluded that "We have no doubt that there is a future for the soda condenser." Oh well, we can't be right all the time.

MORE ON THE HONIGMANN CAUSTIC SODA LOCOMOTIVE: 1885
This article appeared in the Scientific American Supplement for 14th April 1885. It is provided courtesy of Project Gutenberg, so I hope there are no copyright issues. Unfortunately the illustrations are not currently available.
"The Honigmann Fireless Engine."
The invention of a self propelling engine, capable of working without fuel economically and for a considerable time, has often been attempted, and was, perhaps, never before so nearly accomplished as about the time
of the introduction into practical use of Faure's electric storage batteries; but at the present moment it appears that electric power has to give way once more to steam power. Mr. Honigmann's invention of the
fireless working of steam engines by means of a solution of hydrate of soda--NaO HO--in water is not quite two years old, and has in that time progressed so steadily towards practical success that it is reasonable to
expect its application before long in many cases of locomotion where the chimney is felt to be a nuisance.
The invention is based upon the discovery that solutions of caustic soda or potash and other solutions in water, which have high boiling points, liberate heat while absorbing steam, which heat can be utilized for the production of fresh steam. This is eminently the case with solutions of caustic soda, which completely absorb steam until the boiling point is nearly reached, which corresponds to the degree of dilution. If, therefore, a steam boiler is surrounded by a vessel containing a solution of hydrate of soda, having a high boiling point, and if the steam, after having done the work of propelling the pistons of an engine, is conducted with a reduced pressure and a reduced temperature into the solution, the latter, absorbing the steam, is diluted with simultaneous development of heat, which produces fresh steam in the boiler. This process will be made clearer by referring to the following table of the boiling points of soda solutions of different degrees of concentration, and by the description of an experiment conducted by Professor Riedler with a double cylinder engine and tubular boiler as shown in Fig. 2:
| Solution of soda | Boiling point at 1 Bar| Steam pressure (gauge) | 100 NaO HO + 10 H2O | 256 | 40
| 100 NaO HO + 20 H2O | 220.5 | 21
| 100 NaO HO + 30 H2O | 200 | 15
| 100 NaO HO + 40 H2O | 185.5 | 10.2
| 100 NaO HO + 50 H2O | 174.5 | 7.7
| 100 NaO HO + 60 H2O | 166 | 6.1
| 100 NaO HO + 70 H2O | 159.5 | 5.1
| 100 NaO HO + 80 H2O | 154 | 4.2
| 100 NaO HO + 90 H2O | 149 | 3.6
| 100 NaO HO + 100 H2O | 144 | 3.0
| 100 NaO HO + 120 H2O | 136 | 2.2
| 100 NaO HO + 140 H2O | 130 | 1.6
| 100 NaO HO + 200 H2O | 120 | 0.95
| 100 NaO HO + 300 H2O | 110.3 | 0.4
| 100 NaO HO + 400 H2O | 107 | 0.3
| |
Experiment No. 15. [3]
The boiler of the engine, Fig. 2, was filled with 231 kg water of two atmospheres pressure and a temperature of
about 135 degC; the soda vessel with 544 kg of soda lye of
22.9% water and a temperature of 200 degC, its boiling point being about 218 degC. The engine overcame the frictional resistance produced by a brake. At starting the temperature of both liquids had become nearly equal, viz., about 153 degC. The temperature of the soda lye could therefore be raised by 47 degC, before boiling took place, but, as dilution, consequent upon absorption of steam would take place, a boiling point could only be reached less than 218 degC, but more than 153 degC.
The engine was then set in motion at 100 revolutions per minute. The steam passing through the engine reached the soda vessel with a temperature of 100 degC; the temperature of the soda lye began to rise almost immediately, but at the same time the steam boiler losing steam above, and not being influenced as quickly by the increased heat below, showed a decrease of temperature. The difference of the two temperatures, which was at starting 1.3 degC, consequently increased to 7.2 degC, after 17 min., the boiler having then its lowest temperature of 148.8 degC. After that both temperatures rose together, the difference between them increasing
slightly to 9.5 degC, and then decreasing continually. After 2 hours 13 min., when the engine had made 12,000 revolutions, the soda solution had reached a temperature of 170.3 degC, which proved to be its boiling point. The steam from the engine was now blown off into the open air during the next 24 min. This lowered the temperature of both water and soda lye by 10 degC and re-established its absorbing capacity. The steam produced under these circumstances had of course a smaller pressure than before, in this way the engine could be driven at reduced steam pressures until the resistance became relatively too great. The process described above is illustrated by the diagram Fig. 1, which is drawn according to the observations during the experiment.
[Illustration: FIG. 1.]
[Illustration: FIG. 2.]
The constant rise of both temperatures during the first two hours, which
is an undesirable feature of this experiment, was caused by the quantity
of soda lye being too great in proportion to that of water, and other
experiments have shown that it is also caused by an increased resistance
of the engine, and consequent greater consumption of steam. In the latter
part of the experiment, where the engine worked with expansion, the rise
of the temperature was much less, and by its judicious application,
together with a proper proportion between the quantities of the two
liquids in the engines, which are now in practical use, the rising of the
temperatures has been avoided. The smaller the difference is between the
temperatures of the soda lye and the water the more favorable is the
economical working of the process. It can be attained by an increase of
the heating surface as well as by a sparing consumption of steam,
together with an ample quantity of soda lye, especially if the steam is
made dry by superheating.
In the diagrams Figs. 3 and 4, taken from a
passenger engine which does regular service on the railway between
Wurselen and Stolberg, the difference of the two temperatures is
generally less than 10 degC. These diagrams contain the
temperatures during the four journeys a, b, c, d, which are performed with
only one quantity of soda lye during about twelve hours, and show the
effects of the changing resistances of the engine and of the duration of
the process upon the steam pressure, which, considering the condition of
the gradients, are generally not greater than in an ordinary locomotive
engine. It can especially be seen from these diagrams that an increase of
the resistance is immediately and automatically followed by an increased
production of steam. This is an important advantage of the soda engine
over the coal-burning engine, in consequence of which less skill is
required for the regular production of steam power. The tramway engines
of more recent construction according to Honigmann's system- Figs. 5 and
6- are worked with a closed soda vessel in which a pressure of 1/2 to 1½
atmospheres is gradually developed during the process. While the counter
pressure thus produced offers only a slight disadvantage, being at an
average only 1/2 atmosphere, the absorbing power of the soda lye is
materially increased, as shown by the following table, and it is,
therefore, possible to work with higher pressures than with an open soda
vessel. Besides this great advantage, it is also of importance that the
pressure in the steam boiler can be kept at a more uniform height.
[Illustration: FIG. 3.] Note: Figs 3 and 4 appear to refer to Figs 4 and 5 in my diagram above.
[Illustration: FIG. 4.]
TABLE. 100 kg Soda Lye containing 20 parts Water with a corresponding boiling point of 220 degC absorbs Steam as follows:
| Final pressure in condenser | Pressure in steam boiler. | Corresponding temperature | |||
| 0 | | ½ atm. | 1 atm. | 1½ atm. | ||
| 80 kg | 125 kg | 200 kg | 350 kg | 2 atm. | 136.0 deg. C. |
| 65 | 88 | 130 | 190 | 3 | 143.0 |
| 51 | 70 | 98 | 125 | 4 | 153.3 |
| 41 | 58 | 80 | 100 | 5 | 160.0 |
| 34 | 48 | 66 | 80 | 6 | 166.5 |
| 27 | 40 | 55 | 70 | 7 | 172.1 |
| 22½ | 33 | 47 | 60 | 8 | 177.4 |
| 19 | 28 | 41 | 52 | 9 | 182.0 |
| 16 | 24 | 35 | 46 | 10 | 186.0 |
| 12 | 18 | 28 | 35 | 12 | 193.7 |
| 9 | 14 | 22 | 33 | 15 | 200.0 |
| 2 | 8 | 12 | 21 | 20 | 215.0 |
Not the least important part of the process with regard to its economy is
the boiling down of the soda lye in order to bring it back to the degree
of concentration which is required at the beginning of the process. This
is done in fixed boilers at a station from which the engines start on
their daily service, and to which they return for the purpose of being
refilled with concentrated soda lye. It is clear that a closed soda
vessel has produced as much steam when the process is over as it has
absorbed, and the quantity of coal required for the evaporation of water
in concentrating the soda lye can therefore be directly compared with
that required in an ordinary engine for the production of an equal
quantity of steam. The boiling down of the soda lye requires, according
to its degree of concentration, more coal than the evaporation of water
does under equal circumstances, and disregarding certain advantages which
the new engine offers in the economy of the use of steam, a greater
consumption of coal must be expected.
But even at the small installation for the Aix la Chapelle-Burtscheid tramway with only two boilers of four
square meters heating surface each, made of cast iron 20 mm. thick, 1 kg of coal converts 6 kg of water contained in the soda lye into steam, while in an ordinary locomotive engine of most modern construction the effect produced is not greater than 1 in 10. There can be no doubt that better results could be obtained if the installation were larger, the construction of the boilers more scientific, and their material
copper instead of cast iron; but even without such improvements the cost
of boiling down the soda lye might be greatly lessened by the use of
cheaper fuel than that which is used in locomotive engines, and by the
saving in stokers' wages, since stokers would not be required to
accompany the engines.
[Illustration: FIG. 5]
[Illustration: FIG. 6]
Apart from these considerations, the Honigmann engines have the great advantage that neither smoke nor steam is ejected from them, and that they work noiselessly. The cost of the caustic soda does not form an important item in the economy of the process, as no decrease of the original quantities had been ascertained after a service of four months duration. Besides the passenger engine already referred to, which was tested by Herr Heusinger von Waldegg [4] in March, 1884, and which since then does regular service on the Stolberg-Wurselen Railway, there are on the Aix la Chapelle-Julich railway two engines of 45,000 kg weight in regular use, which are intended for the service on the St. Gothard Railway. Their construction is illustrated in Figs. 7 and 9, and other data are given in a report by the chief engineer of the Aix la Chapelle-Julich Railway, Herr Pulzner, which runs as follows:
Wurselen, Dec. 23, 1884.A trial trip was arranged on the line Haaren-Wurselen, the hardest section of the Aix la Chapelle-Julich Railway. This section has a gradient of 1 in 65 on a length of 4 km; and two curves of 250 and 300 meters radius and 667 meters length. The goods train consisted of twenty-two goods wagons, sixteen of which were empty and six loaded. The total weight of the wagons was 191,720 kg, and this train was drawn by the soda engine with ease and within the regulation time, while the steam pressure was almost constant, viz., five atmospheres. The greatest load admissible for the coal burning engines of 45,000 kg weight on the same section is 180,000 kg. Proof is therefore given that the soda engine has a working capacity which is at least equal to that of the coal burning engine. The heating surface of the soda engine, moreover, is 85 square meters, while that of the corresponding new Henschel engine is 92 square meters. On a former occasion I have already stated that the soda engine is capable not only of performing powerful work and of producing a large quantity of steam during a short time, but also of travelling long distances with the same quantity of soda. Thus, for example, a regular passenger train, with military transport of ten carriages, was conveyed on Nov. 6, 1884, from Aix la Chapelle to Julich and back, i.e., a distance of 45 km, by means of the fireless engine. The gradients on this line are 1 in 100, 1 in 80, and 1 in 65, being a total elevation of about 200 meters. For a performance like this a powerful engine is required, and a proof of it can be recognized in the consumption of steam during the journey, for the quantity of water evaporated and absorbed by 4½ to 5 cubic meters soda lye was 6,500 liters. Herr Pulzner, Chief Engineer of the Aix la Chapelle-Julich Railway |
[Illustration: FIG. 7.]
[Illustration: FIG. 8.]
Another certificate concerning the tramway engine illustrated in Figs. 5 and 6 is of equal interest, and runs as follows:
Aix la Chapelle, Jan. 5, 1885. |
Here are some unquestionable results. For nearly a year the first railway engine, and for six months the first tramway engine of this new construction, have been introduced into regular public service, and been open to public inspection as well as to the criticism of the scientific world. They are worked with greater ease and simplicity than ordinary locomotive engines; the economy of their working appears, allowing for shortcomings unavoidably attached to small establishments, to be at least equally great: they do not emit either steam or smoke, and their action is as noiseless as that of stationary engines.
In view of these facts it might be expected that railway managers, who are continually told that the smoke of their engines is a serious annoyance to the public, would be eager to make themselves acquainted with them; it might, in particular, be expected that the managers of the underground and suburban railways of this metropolis would lose no time in making experiments on their own lines--if only by converting some of their old engines into those of the fireless system--and assist a little in the development of an invention, in the success of which they have a tangible interest which is much greater than that of any railway on the Continent, but there is no sign yet of their having done anything.
E., in The Engineer.
[Footnote 3: Zeitschrift d. Vereins Deutscher Ingenieur, 1883, p. 730; 1884, p. 69.]
[Footnote 4: Z.d.V.D.I., 1884, p. 978]
MAGAZINE OF THE ENTIRE LOCAL RAILWAY AND TRAMWAY OF WESER
This enigmatic document (published in Wiesbaden in 1885) is in German and I have not had the time to tranlate it properly, but it contains some interesting material on the Honigmann scheme.
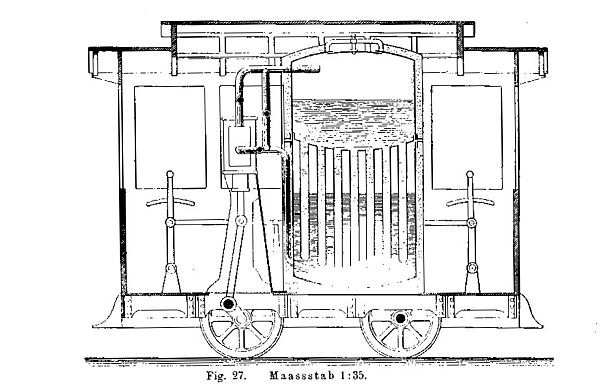 | Left: Project for soda-powered tramcar
|
THE BALDWIN SODA TRAMCARS: 1886
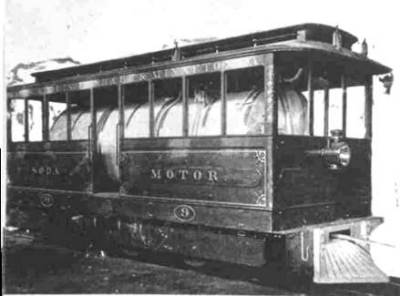 | Left: A soda-powered tramcar.
|
 | Left: The internals of the soda-powered tramcar.
|
 | Left: A report on the Baldwin soda-powered tramcars, from Manufacturer and Builder Volume 21, Issue 6, June 1889.
|
In 1896 the Honigmann principle was proposed for powering submarines while submerged. This sounds like quite a good idea, but it doesn't seem to have come to anything.
(From Hydrogen Peroxide for Power & Propulsion by P R Stokes; a paper given before the Newcomen Society, 14 Jan 1998)
The soda system evaluated:
Advantages:
1) The soda locomotives were silent, there being no exhaust to the outside world. In this respect they were superior to the compressed-air motors.
2) Inferior coal could be used in the fixed installation that reconcentrated the caustic soda.
Disadvantages:
1) Having to lug around the weight of a big tank of caustic soda cannot have helped performance or efficiency. On the other hand, there was no need to carry coal about.
2) Caustic soda can cause nasty burns. A boiler explosion is never a good idea, but adding the prospect of being drenched in hot caustic soda is enough to discourage the most sanguine of prospective passengers.

  
|